Abstract
INTRODUCTION: The clonoSEQ® Assay (Adaptive Biotechnologies Corporation, Seattle, USA) uses next-generation sequencing (NGS) based testing to identify disease-specific immunoglobulin sequences and detect evidence of minimal residual disease (MRD). MRD assessments have been shown to predict progression-free survival and overall survival in non-Hodgkin's lymphoma (NHL) patients. We report MRD results via the commercially available clonoSEQ® assay and its association with durability of response in patients treated with bispecific LV20.19 CAR in our phase 1/2 trial (NCT04186520).
METHODS: We conducted a phase 1/2 single-center prospective trial (NCT04186520) to evaluate the efficacy and safety of LV20.19 CAR T-cells in patients with NHL at 2.5x106 cells/kg dose. LV20.19 CAR T-cells were manufactured onsite at varying lengths of manufacturing (8 vs 12 days) in the CliniMACS Prodigy device with IL7+15 for cell expansion, with the goal of fresh infusion. The commercial clonoSEQ® assay (by Adaptive Biotechnologies) was used to identify and track tumor clonotypes in responding patients at first assessment performed between Day 28-60. We evaluated associations between MRD status and long-term response post LV20.19 CAR-T cell therapy. Descriptive statistics were utilized for baseline characteristics. Survival analyses were calculated utilizing the Kaplan-Meier method and differences between groups were evaluated via the log-rank test.
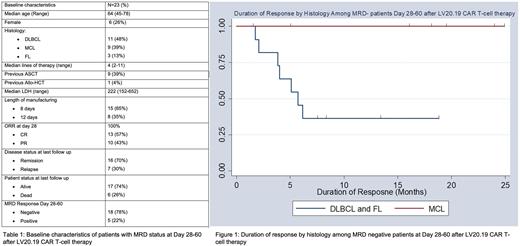
RESULTS: Among the 32 patients enrolled in the trial, there were 26 responders on Day 28. Of these responders, 23 patients had clonoSEQ® B-cell MRD assay done between Day 28-60 post CAR infusion (Table 1). MRD was performed in 11 patients with Diffuse Large B-cell Lymphoma (DLBCL), 3 patients with Follicular lymphoma (FL), and 9 patients with Mantle Cell Lymphoma (MCL). The median age was 64 years (45-74) and the median lines of prior therapy were 4 (2-11).
Of the 23 patients with early MRD status available, the day 28 objective response rate (ORR) was 100% (complete response=57%, n=13 and partial response=43%, n=10). Eighteen patients (78%) were MRD negative at first assessment between days 28-60 and 5 (22%) were MRD positive. Of the 5 patients who were MRD positive, 3 became MRD negative at day 90, 1 patient was non-evaluable due to a non-relapse mortality event, and the remaining patient remains positive but with decreasing MRD status at Day 90. None of the patients with MRD positivity at day 28 have relapsed to date. Among patients who achieved early MRD negativity (n=18), 7 (39%) have relapsed, and all were patients with either DLBCL or FL. No MRD-negative MCL patient has relapsed to date. The duration of response in MRD-negative patients was significantly improved in MCL patients compared to DLBCL/FL patients (Figure 1), p=0.02.
Among the 7 MRD negative patients who relapsed, only 1 patient was detected to have disease relapse by MRD positivity, 2 were detected simultaneously on MRD testing and imaging, while 3 were detected by physical exam or imaging findings and did not have MRD testing. Interestingly, 1 patient had a central nervous system relapse while being MRD negative.
CONCLUSION: Relapse after CAR T-cell therapy remains a significant clinical challenge. New predictors are indicated to determine which responding patients after CAR-T cell therapy are more likely to relapse. Utilizing the commercially available Adaptive clonoSEQ® assay, early MRD assessment was not predictive of long-term clinical outcomes in DLBCL and FL. MRD positive patients maintained durable remission and converted to MRD negativity, while early MRD negative patients continued to relapse. In contrast, MRD negativity post-CAR-T therapy did appear to predict durable clinical remission in MCL patients. Newer assays, including cell-free technologies, may be more informative for non-MCL histologies.
Disclosures
Fenske:Adaptive Biotechnologies: Consultancy, Speakers Bureau; Beigene: Consultancy; Bristol-Meyers-Squibb: Consultancy, Speakers Bureau; CSL Therapeutics: Consultancy; Karyopharm: Consultancy; Kite (Gilead): Consultancy, Speakers Bureau; MorphoSys: Consultancy, Speakers Bureau; Pharmacyclics (AbbVie): Consultancy; SeaGen: Consultancy, Speakers Bureau; Servier Pharmaceuticals: Consultancy; TG Therapeutics: Consultancy, Speakers Bureau; ADC Therapeutics: Consultancy, Speakers Bureau; Astrazeneca: Speakers Bureau; Sanofi: Speakers Bureau. Johnson:Miltenyi Biotec: Research Funding. Hamadani:Medical University of Wisconsin: Current Employment; Incyte Corporation: Consultancy; MorphoSys: Consultancy; Kite: Consultancy; Genmab: Consultancy; SeaGen: Consultancy; Gamida Cell: Consultancy; Novartis: Consultancy; Legend Biotech: Consultancy; Kadmon: Consultancy; ADC Therapeutics: Consultancy, Research Funding, Speakers Bureau; Omeros: Consultancy; Abbvie: Consultancy; Takeda: Research Funding; Spectrum Pharmaceuticals: Research Funding; Astellas Pharma: Research Funding; Sanofi Genzyme: Speakers Bureau; AstraZeneca: Speakers Bureau; BioGene: Speakers Bureau. Shah:Incyte Corporation: Consultancy, Honoraria, Speakers Bureau; Kite Pharma: Consultancy; TG therapeutics: Consultancy; Lilly Oncology: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy; Miltenyi Biotec: Consultancy, Research Funding; Novartis: Consultancy; Epizyme: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal